Introduction
 Sarcopterygii,
or the lobe-finned fishes, includes the coelacanths, lungfishes, fishes
involved in the transition to land, and all tetrapods (mammals,
amphibians, and reptiles [the birds, turtles, crocodiles, and squamates]). The
lobe-finned fishes are Devonian in age and the sister group to Actinopterygii,
or ray-finned fishes. Actinopterygii and Sarcopterygii are nearly of equal size
(c. 30,000 spp. each). Actinopterygii is dominated by the teleost fishes, just as
Sarcopterygii is dominated by tetrapods. In this essay, the focus will be the
non-tetrapod members of Sarcopterygii, as I study fishes; however, it is worth
noting many of the skeletal elements and organ systems of tetrapods originated
in our aquatic sarcopterygian ancestors.
Had actinopterygians been the group to take charge as the vertebrate class
to dominate land, terrestrial vertebrates would look very different. It is likely that we would breathe through
our mouths alone or through our skin, be much smaller, and be hugging the
ground with soft rays holding us up against gravity rather than digits and
wrist bones. It was the advent of internal nostrils, or choanae, in aquatic
sarcopterygians that permitted us to breathe through our noses;
Sarcopterygii,
or the lobe-finned fishes, includes the coelacanths, lungfishes, fishes
involved in the transition to land, and all tetrapods (mammals,
amphibians, and reptiles [the birds, turtles, crocodiles, and squamates]). The
lobe-finned fishes are Devonian in age and the sister group to Actinopterygii,
or ray-finned fishes. Actinopterygii and Sarcopterygii are nearly of equal size
(c. 30,000 spp. each). Actinopterygii is dominated by the teleost fishes, just as
Sarcopterygii is dominated by tetrapods. In this essay, the focus will be the
non-tetrapod members of Sarcopterygii, as I study fishes; however, it is worth
noting many of the skeletal elements and organ systems of tetrapods originated
in our aquatic sarcopterygian ancestors.
Had actinopterygians been the group to take charge as the vertebrate class
to dominate land, terrestrial vertebrates would look very different. It is likely that we would breathe through
our mouths alone or through our skin, be much smaller, and be hugging the
ground with soft rays holding us up against gravity rather than digits and
wrist bones. It was the advent of internal nostrils, or choanae, in aquatic
sarcopterygians that permitted us to breathe through our noses;  | |
| The rare ray-finned fish that can "walk" on land, the mudskipper. Image from http://www.studentsoftheworld.info/sites/animals/shadows.php |
Evolution and
Systematics
Coelacanths
belong to Actinistia (or Coelacanthimorpha), which has a long fossil record
(Mid-Devonian to Late Cretaceous) and that is species rich relative to the two
species extant today (83 valid fossil species in nine worldwide families).
Members of Actinistia are easily recognized by their tri-lobed diphycercal tail
(the vertebral column enters the middle lobe). Known as fossils from both marine
and freshwater deposits, they were thought to have gone extinct over 65 million
years ago, until a living species was discovered in 1938 to much fanfare. (The
discovery of both living species have spectacular stories behind them. See www.dinofish.com)
Lungfishes
are members of Dipnoi (themselves part of the larger group Porolepimorpha, largely
made up of extinct forms). This clade also evolved in Early Devonian freshwaters,
and is represented in the fossil record by more than 100 species in more than
50 genera. Their great fossil record of lungfishes was likely aided by their
ability to estivate. These fishes can protect themselves from drought by
building a mucous-mud cocoon. They enter periods of estivation that in modern
forms can last up to four years; many individuals in the past have expired
waiting for that next rain. These individuals and their cocoons make for spectacular,
if plaintive, fossils. From fossil forms, we see a trend toward the reduction
of bone (in the skull, scales, and fins). Unique plate-like grinding toothplates
easily help place extinct and extant forms as each other’s closest relatives.
Tetrapodomorphs are the intermediate
forms between the first tetrapods to conquer land and their piscine ancestors. They
are all limbed, extinct early Devonian forms, and air-breathers. They include Osteolepimorpha
(rhipidistians), Rhizodontiformes, and Elpistostegalians. It is the
tetrapodomorphs, in particular the Elpistostegalians (which includes Tiktallik) and not coelacanths or
lungfishes, that are the closest relatives to tetrapods.
Physical
Characteristics
Both lungfishes
and coelacanths can reach large sizes, approaching 2 m, although lungfishes are
much more slender bodied. Both groups have a number of derived features that
make each group unique. Coelacanths have a special rostral electroreceptive
organ, a vertebral column that is secondarily reduced, no maxilla, and an
intercranial joint found in many extinct fish lineages but no other living
species. Coelacanths have only external nostrils (no choanae) and a large fat-filled
gas bladder (no lung). These two latter features have been used by some authors
as evidence that these fishes are ancestral to lungfishes (which have both
lungs and choanae), but these primitive features may have more to do with the
current ecology of these animals than their biological history.
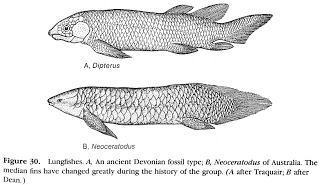
There are three extant families of
lungfishes: Ceratodontidae of Australia, Lepidosirenidae of South America, and
Protopteridae of Africa. Lungfishes are
easily recognized by their continuous rear fins that connect their dorsal,
caudal, and anal fins. The Australian Lungfish
(Neoceratodus forsteri) has a number
of pleisiomorphic morphological features that resemble fossil forms more so
than the other extant lineages. Instead of the tiny worm-like fins of the other
species, the Australian form has broad flat fins, large scales, and unpaired
lungs (versus small scales and paired lungs in the other taxa). Lungfishes
eat both plant and animal material, including ray-finned fishes and
invertebrates.
Reproductive
Biology
Coelacanths are ovoviviparous;
they retain eggs in the body cavity. The young hatch and develop internally. African
and South American lungfishes make nests where females lay eggs, and males
guard the nests. The Australian species lays its eggs on aquatic plants. The
African and South American forms have young with large external gills that
often cause them to be mistaken for salamanders.
Conservation
The
conservation status of most lungfishes is poorly known, but the Australian
lungfish is uncommon, confined to just four rivers in Queensland.
Among
coelacanths, Latimeria chalumnae is
found off the eastern to southeastern coast of Africa and around the Comoros
Islands and Madagascar, and L. menadoensis is only known from Sulawesi, Indonesia. Coelacanths are found at depths beyond the range of most artisanal
fishermen (150 to 253m), but accidental capture occurs frequently enough that
some estimate that as much as 5% of the adult population is captured annually. Coelacanths
aggregate and rest in caves; they may be limited by the number of these sites that
are available.
Significance to
Humans
As Moyle and
Cech state, “probably no single event in the history of ichthyology has
received more public attention than the discovery of the coelacanth (Latimeria chalumnae) in 1938.” The
discovery of this large, deep sea, limbed, fish-link-to-man made for fantastic
headlines. Lungfishes, too, have a
spectacular mix of features that make them popular aquarium fishes. Both
sarcopterygian fish clades are important to humans for their unique position on
the other side of the coin to the vertebrate transition to land.
References
Bemis, W.E,
Burggren, W.W., Kemp, N.E. (1987) The biology and evolution of lungfishes. Alan R. Liss, Inc., New
York.
Carroll,
R.L. 1996. Vertebrate paleontology and evolution. W.H. Freeman. New York.
Helfman,
G.S., Collette, B.B, Facey, D.E., Bowen, B.W. 2009. The diversity of fishes, 2nd
ed. Wiley Blackwell, West Sussex, UK.
Moyle, P.B.,
and Cech Jr., J.J. (2004) Fishes, an introduction to ichthyology, 5th edition. Prentice Hall, New
Jersey.
Musick,
J.A., Bruton, M.N., Balon, E.K. (1991) The biology of Latimeria chalumnae and evolution
of coelacanths. Environmental Biology of
Fishes 32, 1-435.